© Copyright 2020 Foshan Membrane Technology Co., Ltd. All rights reserved. Sitemap
Introduction
The skin is the first barrier for the human body to protect the internal organs against microorganisms or other external hazards1. However, the skin is highly susceptible to damage due to injury or illness. Cutaneous wounds are easily infected, resulting in a massive burden on the healthcare system2. Traditional wound dressing materials such as cellulose, silk, alginate, collagen, and so forth, have no ability to inhibit bacteria colonization or avoid microorganisms’ growth3,4,5. Therefore, there is a need for antibacterial wound dressings to prevent cutaneous wound contamination. Recently, electrospun nanofiber scaffolds have attracted considerable attention in the field of wound dressings due to their unique characteristics, such as high oxygen permittivity, high tensile strength, diverse morphological features, tunable porosity and tailored ability6. Antibacterial ingredients such as antibiotics, metal oxides, and active carbon nanoparticles are being incorporated into the fibrous matrix to aid in the healing of cutaneous wounds7,8.
Poly(ε-caprolactone) (PCL) is a type of biodegradable and biocompatible aliphatic linear polyester that can be synthesized by the ring-opening polymerization of ε-caprolactone. PCL has received considerable attention due to its high toughness, biodegradability, and biocompatibility. It has been demonstrated that electrospun PCL nanofibrous scaffolds can be utilized for wound dressing applications9,10,11. The native porous structure characteristics of PCL scaffolds can mimic the skin's extracellular matrix (ECM) structural properties while also providing high oxygen permeability. In order to confer antibacterial capabilities on the PCL nanofibrous scaffolds, different types of metal oxides or metals have been introduced into the PCL matrices12. Zhu et al. found that the insertion of silver (Ag) and magnesium (Mg) ions into gelatin/polycaprolactone (GT/PCL) could endow the nanofibers with antibacterial activity as well as pro-angiogenesis function, which benefited for skin wound repair13. Ghiyasi and his colleagues discovered that the hybrid scaffolds consisting of Urtica dioica, ZnO nanoparticles and PCL had good antibacterial activity against E. coli and S. aureus14. In addition, the nanofiber of the hybrid scaffold exhibited good biocompatibility and cell adhesion to fibroblast L929 cells in vivo tests. Ekram et al. demonstrated that the presence of zinc chloride (ZnCl2) reduced the diameter of the PCL/ZnCl2 nanofibers while increasing the degradation rate and mechanical properties15. Moreover, the antibacterial composite nanofiber was found to greatly boost the proliferation of stem cells. Trcin et al. found that the PCL scaffolds containing TiO2 nanoparticles could provide statistically significant antimicrobial activity against different types of bacteria16. Furthermore, the PCL/TiO2 scaffolds with a maximum porosity of 93%, on the other hand, were found to be capable of supporting the adhesion and proliferation of limbal stem cells.
MXene is a new family of two-dimensional (2D) materials that integrates the transition metals M (Ti, Cr, V, Nb and Mo etc.) with huge amounts of X (carbides, nitrides, or carbonitrides) by removing the A-element in the MAX phase17,18. MXene (Ti3C2TX) exhibits superior biocompatibility and antibacterial efficiency against both Gram-negative and Gram-positive bacteria than graphene oxide due to its ultrathin structure and unique physiochemical properties19,20. Awasthi et al. found that the addition of MXene into PCL nanofibers maintained good biocompatibility in vitro with ibroblasts (NIH-3T3) and preosteoblasts (MC3T3-E1) cell lines21. In addition, the presence of Ti3C2TX nanosheets contributed to reducing the diameter and improving the morphology of PCL/Ti3C2TX nanofibers. However, no data on the antibacterial activities of PCL/Ti3C2TX nanofibers have been reported to far.
Herbal extracts have been widely utilized to cure various diseases since ancient times. Plant-derived phytochemicals can serve as potential antibacides with fewer side effects. Baicalin, a flavonoid extracted from the Chinese herb Scutellaria baicalensis, has been regarded as a multitherapeutic agent in the field of biomedicine22. It shows various positive benefits on wounds, including anti-oxidative, anti-bacterial, and anti-inflammatory properties23,24. However, little research has been performed to date on the use of baicalin in electrospun fibers for wound dressings.
In this work, MXene (Ti3C2TX) was first exfoliated to obtain Ti3C2TX nanoflakes. Then the resulting Ti3C2TX nanoflakes were incorporated into the PCL matrix with herbal extraction baicalin by electrospinning. The morphology, thermal stability, hydrophilicity, and mechanical properties of the electrospun nanofibers consisting of PCL/Ti3C2TX/baicalin ternary composites were investigated. The addition of Ti3C2TX and baicalin was expected to have synergistic effects on improving the wound dressing's antibacterial performance against gram-positive bacterial S. aureus. Furthermore, the biocompatibility in vitro of this wound dressing was also evaluated by using rat skeletal myoblast L6 cells.
Materials and methods
Materials
Poly(ε-caprolactone) (PCL, CapaTM 6800) with a mean molecular weight of 80,000 was obtained from Weibo Chemical Co., Ltd. (Guangzhou, China). MXene (Ti3C2TX) with 400 meshes was supplied by Beike 2D materials Co., Ltd. (Beijing, China). Baicalin (purity > 95%) was supplied by Macklin Biochemical Co., Ltd. (Shanghai, China). Chloroform and dimethylformamide (DMF) were purchased from J&K (Beijing, China).
Preparation of electrospun PCL composite nanofibrous membranes
The exfoliation of Ti3C2TX was performed by high energy ball milling using a Miqi YXQM-1L planetary micromill (Changsha, China). Ti3C2TX (4 g) and zirconium oxide milling balls (70 g) were placed in a 250 ml grinding bowl and performed at 600 rpm for 4 h. Then the exfoliated Ti3C2TX was removed from the grinding bowl with DI water, followed by sonication in DI water for 30 min to exfoliate completely. Finally, the resulting Ti3C2TX nanoflakes were centrifuged at 3500 rpm for 10 min, and the obtained suspension was freeze-dried overnight.
The electrospun nanofibers of the PCL/Ti3C2TX/baicalin ternary composite were prepared using a commercially available electrospinning machine (TL-Pro, Tongli Weina Co., Ltd., Shenzhen, China), as shown in Fig. 1. The formulations of PCL based nanofibers are shown in Table 1. The concentration of PCL was fixed at 100 mg/mL in a mixed chloroform/DMF (8:2, v/v) solution. Subsequently, the desired amount of Ti3C2TX and baicalin was added into the PCL solution with vigorous stirring. The prepared PCL solution was loaded into a 10 mL plastic syringe with a metal capillary needle (0.50 mm inner diameter, and 30 mm length). The applied electrospinning voltage was fixed at 15 kV and the flow rate was kept at 1 mL/h. The obtained nanofibers were subsequently placed in a vacuum oven at 50 ℃ for 6 h to remove the remaining solvent.

The morphology of Ti3C2TX and the prepared nanofibers was observed by a scanning electron microscope (SEM, FEI Quata 250, USA). Prior to observation, the specimens were sputtered with a thin layer of gold to avoid charge accumulation.
The thickness of Ti3C2TX nanoflakes was measured by an atomic force microscopy (AFM, VEECO Multimode V, USA) with tapping mode.
The X-ray diffraction patterns were conducted on a grazing-incident XRD (Rigaku SmartLab) with Cu Kα at 45 kV. The scanned angle (2θ) ranged from 5° to 60°.
The Fourier-transform infrared (FT-IR) spectra were performed with a Perkin Elmer FTIR-100 spectrometer (USA) with a collected wavenumber range of 500–4000 cm−1.
The thermal stability was evaluated by a thermogravimetric analyzer (TGA, Netzsch TGA-209F1). The specimens were heated from room temperature to 600 ℃ at a ramping rate of 10 ℃/min.
The wettability of the nanofibers was evaluated via the measurement of the water contact angle using a See System E instrument (Advex Instruments, Czech Republic).
The standard broth microdilution method was applied to determine the MIC value of baicalin toward S. aureus as described in the previous reports25,26. Briefly, the S. aureus was inoculated and grown in a broth subculture inside a flask. The bacteria were then incubated at 37 °C for 24 h. Then, the bacterial concentration was adjusted to a density of 1.0 × 106 CFU/ml. The baicalin solution, with a concentration ranging from 1 to 1024 mg/ml, was added into the S. aureus solution to observe the bacteria’s growth. The lowest concentration, at which no visible bacterial growth was observed in the plate, was considered as the MIC.
The antibacterial properties of the PCL based nanofiebers against S. aureus were evaluated by a standard “SNV 195920-1992” evaluation model26. The evaluation standard of inhibition zone is summarized in Table S1. The Firstly, 100 µL of 108 CFU/mL bacteria suspension was spread on an LB agar plate, and then the nanofibrous membrane samples with a diameter of 1.0 cm were placed on the surface of the agar. The bacteria suspension with the PCL-based films were incubated at 37 °C, followed by the digital images of the PCL-based nanofibrous membranes on the agar plate with bacteria were recorded at 24 h, 72 h, and 120 h, respectively. All samples were tested in triplicate.
The cytotoxicity of the nanofibers on rat skeletal myoblast L6 cells was evaluated using the CCK-8 method with a leaching pattern. The sterilized nanofibers extract solutions (10 mg/mL) were prepared by immersing the dried nanofiber in medium for 12 h at 37 ℃ with ultrasonic extraction. The L6 cells were seeded in a 96-well plate at a density of 5000 cells/well, and pre-cultured for 24 h before replacing the culture medium with the fresh medium and extract solutions to make the final sample concentration of 0.2 mg/mL, 1 mg/mL, and 5 mg/mL. Each sample to be tested (PCL-0, PCL-1, PCL-2, PCL-3, and PCL-4), blank control (culture medium) and positive control (culture medium and cell) were incubated for 48 h and repeated four times. The results were recorded as the absorbance at 450 nm through an ultraviolet spectrophotometer by the following formula:
$${\text{Cell Growth Rate }}\left( {{\text{RGR}}} \right)\, = \,\left( {{\text{Test}}_{{{\text{OD45}}0}} -{\text{ Blank}}_{{{\text{OD45}}0}} } \right)/\left( {{\text{Positive}}_{{{\text{OD45}}0}} -{\text{ Blank}}_{{{\text{OD45}}0}} } \right)\, \times \,{1}00\% .$$
All experiments were carried out in triplicate. The data was analyzed by the SPSS software (IBM Analytics, USA). Significance of all the statistical tests was predetermined at P < 0.05. Results were expressed as mean ± standard deviation (SD).
Results and discussion
Characterization of MXene nanoflakes
In Fig. 2A, the pristine MXene (Ti3C2TX) shows a typical organ-like structure with gaps of tens of nanometers wide. The exfoliated Ti3C2TX nanoflakes in Fig. 2B show that the layers are clearly separated from each other. In addition, some small nanoflakes in the range of tens of nanometers are observed due to the grinding. The EDX elemental mapping images in Fig. 2C confirm the presence of Ti, C, and O, which is consistent with the chemical structure of Ti3C2TX20. The appearance of F on the surface of Ti3C2TX nanoflakes is due to the remaining F element after etching with LiF/HCl17. In the AFM image in Fig. 2D, it is observed that the exfoliated Ti3C2TX nanoflake has a thickness of 1.5 nm, which is similar to the reported results27,28. The XRD patterns of pristine Ti3C2TX and exfoliated Ti3C2TX nanoflakes are shown in Fig. 2E. It is noted that the characteristic peak at 6.9° in pristine MXene indicates the interlayer spacing of 1.28 nm. In addition, the prominent peaks at 9.4°, 19.1°, 34.0°, 38.7°, 41.7° and 44.9°, which correspond to the diffraction of (002), (004), (101), (008), (104), and (105) planes of Ti3C2TX, respectively29. As for the exfoliated Ti3C2TX nanoflakes, the characteristic peak shifts from 6.9° to 5.4° due to the exfoliation. In addition, this peak becomes board and weak, which is ascribed to the ball milling and exfoliation reducing the size of the nanoflakes and enlarging the interlayer spacing.

The SEM images of PCL nanofibrous membranes are presented in Fig. 3. It is noted that the nanofibers of PCL-0 with a mean diameter of 269 nm (Fig. 3B) have a smoother surface and non-beaded structures. With the addition of 3 wt% Ti3C2TX nanoflakes, the mean diameter of PCL-1 decreases from 269 to 217 nm as compared with that of PLA-0. In Fig. 3F,H,J, the mean diameters of PCL-2, PCL-3 and PCL-4 are 254 nm, 223 nm, and 210 nm, respectively. It is believed that the presence of conductive Ti3C2TX nanoflakes can increase the charge density of the electrospinning solution due to the polar groups on their surface, thus enhancing the electrostatic force of the applied electric field and reducing the diameter of the nanofibers30,31. On the other hand, the introduction of baicalin has little effect on the diameter of the nanofibers. However, the surface of the PCL nanofibers containing baicalin is relatively smoother than that of PCL-1. It is speculated that baiclin is a type of small molecule with many hydroxyl groups that can reduce the viscosity of the electrospinning solution and react with the polar groups of Ti3C2TX nanoflakes to form hydrogen bonds, resulting in the homogeneous dispersion of Ti3C2TX nanoflakes in the PCL matrix.

The typical tensile stress versus strain curves for PCL-based nanofibrous membranes are shown in Fig. S1A, and the corresponding tensile properties are summarized in Fig. S1B. It is noted that the pure PCL membrane shows high ductility (elongation at break of 305%), which is consistent with other published works32,33. For PCL-1, the addition of Ti3C2TX nanoflakes results in strong reinforcing effects, increasing the tensile modulus significantly. Although the abundant polar groups on the surface of Ti3C2TX nanoflakes can react with the ester bonds of PCL, the inhibition effects of the rigid nanosheets will be more profound, lowering the plastic flow ability of PCL molecular chains34. On the other hand, the PCL-2 shows a clear decrease in both elasticity and strength. In Figure S1B, the elongation at break and tensile strength of PCL-2 are reduced to 178% and 2.58 MPa, respectively. This is because baicalin is a small molecule that has a plasticizing effect on PCL. As for PCL-3, the tensile strength of the samples improved obviously, with a little sacrifice in elongation at break as compared with those of PCL-2. It is speculated that the presence of Ti3C2TX nanoflakes can react with baicalin to form hydrogen bonds to some extent. With the further increase in the baicalin content, the tensile properties of PCL-4 deteriorate progressively, indicating the baicalin exceeds the reaction sites of Ti3C2TX nanoflakes.
The FT-IR spectra of PCL-based nanofibrous membranes are presented in Fig. 4. It is clearly observed that the two characteristic peaks located at 2943 cm−1 and 2863 cm−1 of PCL-0 correspond to the stretching bands of CH2 groups. The absorption peak at 1737 cm−1 is ascribed to the stretching of the C=O groups, while the peaks at 1294 cm−1 and 1184 cm−1 belong to the asymmetric and symmetric stretching of the C–O–C groups, respectively. In addition, the characteristic peak at 1243 cm−1 denotes the CH3 vibrations, and 1045 cm−1 belongs to C–O stretching and C–H bending35. With the incorporation of Ti3C2TX and baicalin, no obvious change is observed in the FT-IR spectra. The ternary composites show a weak and broad peak between 3600 and 3300 cm−1, which is ascribed to the overlapping of the O–H polar groups on the surface of Ti3C2TX and the O–H stretching vibration of baicalin36. Furthermore, there is a new characteristic peak at 1609 cm−1 that corresponds to the stretching vibration of C=C of phenyl groups from baicalin37.

The thermal stability of PCL-based nanofibrous membranes was measured by TGA, as shown in Fig. 5. The corresponding data, including the initial weight loss temperature (T10, the temperature at 10% weight loss), the peak weight loss temperature (Tp, the temperature at maximum weight loss rate), and char residues at 600 ℃ are listed in Table 2. It can be observed that PLA-0 shows a distinct weight loss stage from 300 to 420 ℃, which was associated with the pyrolysis of the chemical bond cleavage of PCL chains38. The thermal decomposition curve of PCL-1 shifts to a higher temperature, indicating the presence of Ti3C2TX can increase the thermal stability of PCL nanofibers. According to Table 2, the T10 and Tp of PCL-1 increases from 361.8 to 365.8 ℃ and from 403.2 to 415.7 ℃, respectively, as compared with that of PLA-0. This is due to the Ti3C2TX nanoflakes serving as a thermal barrier to protect the underlying PCL matrix. In the case of the PCL/Ti3C2TX/baicalin ternary nanofibers, the thermal decomposition curves can be roughly divided into two major stages. The initial weight loss stage occurs in the range of 200–370 ℃ due to the thermal decomposition of baicalin. The second stage occurs at around 370–420 ℃, which is related to the pyrolysis of PCL chains. In addition, the T10 and Tp of PCL-4 show a decreasing tendency as compared with those of PLA-0, which is ascribed to the low thermal stability of baicalin.
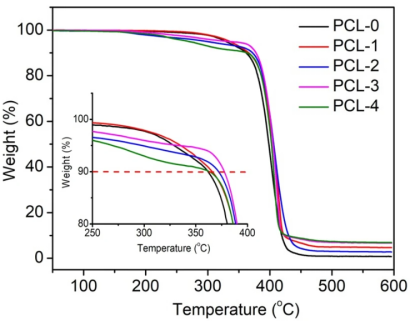
Figure 6 depicts the XRD patterns of PCL-based nanofibrous membranes. As shown in Fig. 6, the PCL-0 has three significant diffraction peaks at 2θ = 21.3°, 22.0° and 23.7°, which correspond to the (110), (111), and (200) planes, respectively39. In addition, there is also a relatively weak and broad peak at 11.8° in the PCL-0 pattern. The above results indicate that the pristine PCL is in a semi-crystalline state. In the XRD pattern of PCL-1, one additional peak appears at 2θ = 6.4°, which is attributed to the (002) plane of Ti3C2TX20. After the introduction of baicalin, the diffraction angle of PCL at 2θ = 11.8° becomes weaker and shifts to a lower angle, indicating that the physical interaction between PCL and baicilin is altered.

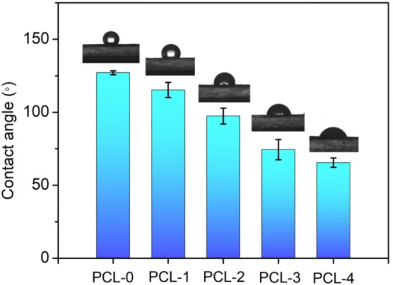
The water contact angles (WCA) of the membrane surfaces were measured to evaluate the hydrophilicity of the PCL-based nanofibrous membranes, as shown in Fig. 7. It is observed that PCL-0 has a hydrophobic surface with a WCA of 127.1° ± 1.3°, which is consistent with previous reports40,41. With the addition of Ti3C2TX nanoflakes, the WCA of PCL-1 shows a slight decrease (115.3° ± 5.2°) due to the abundance of −OH groups on the surface of Ti3C2TX. When baicilin was introduced into the PCL matrix, the surfaces of the PCL nanofibrous membrances shifted to hydrophobicity. The contact angles for the PCL-2, PCL-3, and PCL-4 are 82.4° ± 5.4°, 74.4° ± 6.9°, and 65.5° ± 3.2°, respectively. It can be attributed to the fact that baicalin contains a large number of hydrogel groups that increase the hydrophilicity of the electrospun fibers.
Baicalin is the main antibacterial constituent of PCL-based membranes, which has broad-spectrum antibacterial activity, especially against Gram-positive bacteria like S. aureus42. In this work, the MIC of baicalin against S. aureus was found to be effective at 32 mg/ml, which is similar to the reported literatures43,44. All PCL-based nanofibrous membranes were selected for a standard antibacterial test by the preincubation plate pouring method. In Fig. 8, the bacterial colonies appear clustered around the PCL-0 and PCL-1 nanofibrous membranes after 72 h in the detailed pictures, and more remarkable clustered colonies are observed after 120 h. This is due to PCL having no antibacterial ability, which is consistent with previous report45. On the other hand, PCL-2, PCL-3, and PCL-4 show obvious bacteriostatic activity at 120 h incubation time (The medium dried completely after 120 h, and further inhibited bacterial growth). It can be ascribed to the presence of baicalin can interrupt the formation of α-heptamer, hindering the cell lysis activity of α-Hemolysin of S. aureus46. In addition, it is observed that the surrounding areas of PCL-3 and PCL-4 exhibit a light yellow color at 24 h, indicating the addition of Ti3C2TX nanoflakes contributed to the spread of baicalin from the nanofibers. That is because baicalin has poor solubility in aqueous solution47.

The cytotoxicity testing was performed by MTT assays so as to evaluate the biocompatibility of the as-prepared PCL-based nanofiber membrances. It is usually to measure the cell density after the cells have been exposed to the nanofibers’ leaching liquor for 48 h to confirm the cytocompatibility of the material. In Fig. 9, it is noted that all groups show no significant cytotoxicity at different concentrations. The cell viability of all test groups modestly declines as the concentration increases. The group with the lowest survival rate remains close to 100% at the highest concentration (5 mg/mL), which suggests that the PCL/Ti3C2TX/baicalin ternary nanofibrous membranes can be utilized as safe wound dressings.

Conclusions
In this paper, the electrospun membrances based on PCL/Ti3C2TX/baicalin ternary composites were prepared for wound dressing applications. The SEM observation showed that the presence of Ti3C2TX nanoflakes could decrease in the diameter of the nanofibers due to the increase in the charge density of the electrospinning solution. The PCL nanofiber membrances containing 3 wt% Ti3C2TX flakes and 5 wt% baicalin had the smallest mean diameter of 210 nm. The thermal stability of the composite nanofibers was improved due to the barrier effect of Ti3C2TX nanoflakes. The addition of Ti3C2TX and baicalin could enhance the hydrophilicity, contributing to the release of baicalin from the nanofibrous membranes. Furthermore, the addition of baicalin could endow the PCL/Ti3C2TX/baicalin ternary nanofibrous membranes with good antibacterial properties. The cytocompatibility test confirmed that all of the PCL-based nanofibrous membranes had good compatibility. The antibacterial PCL/Ti3C2TX/baicalin ternary nanofibrous membranes have great potential for wound addressing applications.
Article Source:https://www.nature.com/articles/s41598-022-13141-0