© Copyright 2020 Foshan Membrane Technology Co., Ltd. All rights reserved. Sitemap
Peripheral nerve injuries vary among patients. Cell-based or acellular nerve conduits have a high peripheral nerve regeneration potential. However, they are not ideal for complicated nerve differentiations and are prone to unclear effects of cytokine interactions. Delayed nerve axon regeneration could be ascribed to a lack of regulation of the regenerative microenvironment. Electrical stimulation influences nerve regeneration by regulating sensitive targets. Here, a piezoelectric nerve conduit was developed using a composite of PCL/ZnO nanofiber (PZNF) via electrospinning. Endogenous piezoelectric stimulation from PZNF facilitated sciatic nerve regeneration. This material inherits polycaprolactone characteristics and is a biocompatible material. It generated stable and desired endogenous electrical stimulations. PZNF demonstrated faster and superior sciatic nerve repair in vivo than polycaprolactone nanofiber and nerve bridging in situ. Piezoelectric stimulation of PZNF could substantially increase nerve growth factor/vascular endothelial growth factor expression. Furthermore, the PZNF considerably promoted rapid nerve repair and shortened function recovery (within 4 weeks) in vivo. Moreover, an increase in growth factor receptor-bound protein-2 (GRB2) expression activated downstrem pathway: RAS/MAPK pathway after piezoelectricity stimulation, indicating that GRB2 may be an electrically sensitive protein and a hint protein of electrical stimulation-induced regeneration. This study offers a novel strategy of applying piezoelectric stimulation in rapid peripheral nerve regeneration.
Peripheral nerve injury, which is typical in all types of trauma, usually causes loss of motor and sensory functions [1]. Over 40% of limb nerve injuries are caused by sciatic nerve injuries [2]. The major complaints of patients with such injuries are persistent and untreatable pain, muscle dysfunction, and feeling disability [3]. Severe peripheral nerve injuries such as sciatic nerve defects lead to irreversible tissue atrophy. Simultaneously, continuity loss and biochemical transportation failure may cause limb dysfunction and nerve necrosis, thereby burdening patients [4], [5], [6].
Clinically, the basic principle of sciatic nerve injury is to have a terminal to bridging without any tension for successful nerve reconstruction [7], [8]. In addition, biological peripheral nerve regeneration plays a critical role after physical bridging [9]. Wallerian degeneration is triggered by injury with several measures of nerve lesions, which may lead the distal axon to nerve regeneration [10]. Moreover, the formation of Schwann cell cords is key to successful axon regeneration [11]; however, the recovery condition is harsh and the nerve regeneration process entails complicated circumstances. Previously, a shorter gap impeding reinnervation or reconnection between two injured axon terminals (ends) was mended to achieve axon regeneration (6–12 weeks), resulting in a higher success rate of nerve regeneration [12]. Numerous nerve repair strategies, such as nerve grafting, bioactive coating, and cytokine and stem cell loading on biodegradable materials and decellularized nerve conduits, have been developed for peripheral nerve regeneration [13], [14], [15], [16], [17]. However, owing to the complexity of nerve regeneration, these materials have difficulties in clinical application with unknown adverse effects and biosafety issues.
Electrical stimulation represents a new era of nerve regeneration [18], [19]. During nerve regeneration, a consistent electrical stimulation attracts migrating Schwann cells and accelerates nerve reinnervation [20]. In vitro electrical stimulation has positive effects on the proliferation of Schwann cells [21]. Upregulated expression of nerve growth factor (NGF) is observed during the process because of in vitro stimulation of Schwann cells [21]. Several materials such as polypyrrole and polystyrenesulfonate have positive effects on peripheral nerve injury [22]. Stable and continuous electrical stimulations are required to accelerate tissue healing and regeneration [19], [23]. However, such materials usually require external electrical stimulations with invasive implantation, inevitably causing difficulty in mobility, limiting their clinical application. In addition, internal electrical stimulations have poor efficiency and unstable electricity output.
Recently, electrical stimulation from piezoelectric materials has shown potential as an internal electric generator for peripheral nerve regeneration [24], [25]. Piezoelectric materials possess electrical charges on their surface, which can serve as electric reservoirs for electrical stimulation [26]. Owing to the possibility of ultrasound-triggered potential changes on the surface, piezoelectric composite films from polyvinylidene fluoride (PVDF) as cell harvest sheets have shown the ability to influence cell differentiation by electrical stimulation [27]. PVDF and its copolymer with trifluoroethylene (PVDF-TrFE) have been demonstrated to be promising biomaterials for supporting nerve regeneration due to electrical stimulation [24]. The use of electroactive biomaterials such as piezoelectric biomaterials is a potential strategy to control or influence cell fate for tissue regeneration by designing specific electricity generator [28]. The path to nerve regeneration with piezoelectrical stimulations has been elucidated by several studies. Based on cell-traction-triggered ECM, such as electrospun PVDF, it has been proved that electrical stimulation with ECM-like cell microenvironment modification could induce neuron-like differentiation in BMSCs [29]. Using nanoscale stripe arrays, Xiaodi et al. [30] designed a surface-modified piezoelectric PVDF for neuron-like induction of stem cells. Another critical point to consider in nerve regeneration is time. Following nerve injury in rats, the damage response has event peaks from 20 min to 3 weeks [31]. Subsequently, the repair events decrease. Therefore, to improve nerve repair, it is important to shorten the axon reconnection time to capture event peaks. Although several materials with electrical stimulation can be used in tissue engineering strategies for nerve repair (8–12 weeks), it is difficult to screen out piezoelectric materials with good electrical output that lasts long, decays slowly, and is relatively stable, with faster axon reconnection. Thus, there is a need to induce peripheral nerve regeneration under piezoelectric stimulation.
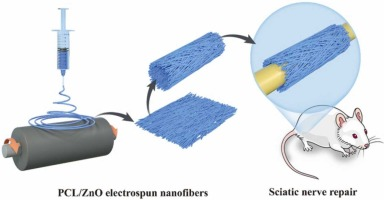
In this study, aligned nanofiber-mat-based nerve conduits were designed and fabricated via electrospinning. It is well known that zinc oxide (ZnO), which has wide applications in nanomaterials, has optical, semiconducting, and piezoelectric properties [32], [33]. The piezoelectric property of ZnO has potential application for endogenous electrical stimulation in peripheral nerve regeneration [34]. Although there is a controversy about the toxicity of ZnO nanoparticles(ZnO NPs), the concentration-dependent toxicity indicates a feasible strategy of concentration control to avoid toxic effects.[35], [36]A composite nanomaterial with a ZnO generator and electrospun polycaprolactone (PCL) was prepared in this study. Meanwhile, different ZnO wt% toxicity effects to Schwann has been carefully performed to monitor a safe ZnO wt% for study. This PCL and ZnO composite can deliver stable electrical stimulations in vivo and in vitro. Previously, a composite of PCL nanofibers with ZnO was applied in hard tissue regeneration, such as bone formation [37], [38], [39]. The nanofibrous structures of PCL have been proved as a novel environment for Schwann cell migration and axonal regeneration [40], [41], [42]. Additionally, PCL biodegradation time ranges from several months to years, which is conducive to nerve repair requirements [43], [44]. For another requirement to conduit repair, stable and sufficient strength of bridging is another important factor influencing peripheral nerve regeneration [45]. Owing to its mechanical properties, PCL tensile strength (around 10 MPa) could meet the repair requirements in nerve defect model [46]. Here, we present the first application of this PCL/ZnO nanofiber (PZNF) for peripheral nerve regeneration. PZNF inherits the advantages of electrospun materials for promoting cell proliferation owing to its surface morphology [47]. This endogenous piezoelectricity generator delivered electrical stimulations transferred from a mechanical power, with good mobility and less traumatic implantation. Meanwhile, the reconnection time of the regenerated axon was sharply shortened (within 4 weeks) compared with the traditional recovery time (8–12 weeks). It was hypothesized that PZNF could serve as a stable endogenous piezoelectric material (Fig. 1).
Polycaprolactone (PCL, Mn = 8 × 105) was purchased from Sigma-Aldrich Co., Ltd. (USA). ZnO powder (30 ± 10 nm) was ordered from Shanghai Macklin Materials Co., Ltd. (China). Hexafluoroisopropanol (HFIP) was procured from Shanghai Fluoro Co., Ltd. (China).
First, a complex solvent was prepared by constantly stirring the mixture of ZnO powder and HFIP. ZnO was completely dissolved via ultrasonic dispersion for 2 h. Second, PCL was dissolved in the solvent at 60 °C for approximately 2 h for the
To assess morphological piezoelectrical characteristics, transmission electron microscopy images of ZnO nanoparticles were obtained with a condensed structure (Fig. S1A, Supporting Information). The typical crystal structure of hexagonal wurtzite was observed (Fig. S1B), which indicated the piezoelectricity of ZnO. Nanofiber mats with different concentrations of ZnO are shown in Fig. 2A. The diameter of the nanofibers was not affected by different concentrations of ZnO. Notably, excessively
We obtained a biodegradable polymer film with an inorganic powder (ZnO) via electrospinning under biosafe concentrations; the film can consistently generate piezoelectricity and provide endogenous electrical stimulation. This material considerably shortened the duration of sciatic nerve repair (within 4 weeks), with functional recovery and physical continuity. The number of myelin sheaths increased significantly. Furthermore, the recruitment of Schwann cells (S100) for nerve filament (NF200)
Article Source:https://www.sciencedirect.com/science/article/abs/pii/S2211285522004001