© Copyright 2020 Foshan Membrane Technology Co., Ltd. All rights reserved. Sitemap
In recent years, healthcare and treatments have trended towards an individualized approach—being able to assess and meet the needs of specific individuals. As healthcare focuses on improving the wellbeing of individual patients, arises the need for the development of sensors that are capable of detecting specific biomarkers or chemical markers indicative of a user's health [1], [2], [3]. Such monitoring informs healthcare and treatment-related decisions and is capable of detecting diseases in earlier stages. Enhanced and earlier detection of illnesses, including malaria [4], [5], [6], various cancers [7], [8], [9], among many others, improves patient outcomes by facilitating treatment during earlier stages—a key for remediating any ailment. A recent development, however, has been the inclusion of nanofibers for these sensing methods.
For the purposes of this survey of recent developments, nanofibers (NFs) are considered to include systems in which the diameter of the fibers is below 1 µm, with a majority being smaller than 500 nm. Nanofibers (NFs) are unique in their one-dimensional structure, as such fibers have large aspect ratios. Such NFs have diameters from 1 to 1000 nm but can have a length that spans several centimeters [10]. Broadly, NFs typically have an extremely high aspect ratio, making them orders of magnitude longer in one direction than they are wide. For example, a fiber could be 5 cm in length, yet only have a diameter of 500 nm. Therefore, the ratio of length to diameter is 100,000. While this can vary largely depending on the NF structure, all NFs are highly one-dimensional by their own definition. These characteristics enable NFs to have a high surface area to volume ratios, allowing for a greater sensing area while still remaining of low overall volume. Due to this, many properties are altered with the use of NFs. One of the main justifications for the utilization of NF-based systems is that the properties on the micro- and nano-scales differ from those of bulk materials [11]. Generally, common types of NFs include carbon nanofibers (CNFs), carbon nanotubes (CNTs), natural & synthetic polymers, and inorganic materials [12], [13], [14], [15], [16]. All structures satisfying the definition set forth of NFs will be considered, including CNTs and biological (cellulose-based) fibers, yet not all systems may be widely used in the survey of literature. When evaluating NFs, many parameters are relevant in their characterization, including the material choice, diameter, diameter distribution, alignment, and functionalization.
To focus the scope of this review, however, only immunosensing applications that implement nanofibers will be considered. While there have been many recent advances in the fields of tissue engineering, healthcare diagnostics, and immunosensing, not all of the fields could be considered in this review. Prior reviews have been written with different foci than this one, including electrochemical immunosensors [17,18], biomarkers for diagnosis of Alzheimer's disease [19,20], breath detection of biomarkers [21,22], among many others. Additionally, NFs have many more uses than just sensing. They have been utilized for drug delivery, wearable devices, energy storage, and many other specific use cases [23]. There have been previous reviews on such related topics, including NFs for biomedical therapeutics [24], drug delivery applications [25], and wearable electronics [26]. Therefore, only the developments of nanofibers for immunosensing and disease detection applications will be evaluated in this review.
A specific subset of nanofiber-based sensors is immunosensors. Immunosensors, fundamentally, are the refinement of immunoassays. Immunoassays have been used for decades as a means of detecting specific biomarkers within complex samples, such as serum or other bodily fluids [27]. The underlying mechanism is based on the immune response in living systems, as antibodies are typically immobilized to the testing substrate (such as a microtiter plate). Then, the complex samples are brought into contact with the antibodies. Here, antigens that are produced as a result of an immune response bind specifically to the immobilized antibodies. After this, multiple pathways can be followed, but all allow for a signal to be generated from the specific binding of the antigen to the antibody that occurred (such as a colorimetric response [28], fluorescent response [29], or measurable electrical response [17]). This detection mechanism relies on the specific antigen-antibody interactions. Immunosensors, broadly, implement antibodies as recognition elements for the detection of target molecules [30,31]. One common application of immunosensors is the enzyme-linked immunosorbent assay (ELISA), and is used for the detection of peptides, proteins, antibodies, and other biomarker molecules [32], [33], [34], and has been used since 1971 [35]. ELISA can be improved with the addition of NFs and has been shown to increase sensitivity and reduce processing time [36]. NFs, in conjunction with immunosensing techniques, allows for a greater surface area and for the entire sensing apparatus to be contained into a signal measuring device. A standard methodology for NF applications that implement antibodies as recognition elements functionalize the recognition molecules to the fibers, which become immobilized and are thus stabilized [37], [38], [39].
Recent progress has been made in the field of NFs for immunosensing and will be organized by the function of the fibers. Most commonly, this will be for the purpose of disease detection, as much work in recent years has contributed to this goal. Mainly, this will focus on malaria detection, cancer detection, and pathogenic bacteria detection, as research into these fields is ongoing and aims to alleviate common causes of death and illness for many. All of the work discussed here within will be proof-of-concept studies being conducted at the research level. While the ultimate goal is for the development of clinical platforms, little commercial success has been demonstrated. Many developments require extensive translation to commercially viable and scalable products. In 2011, a patent was filed for the detection of biologically active analytes with NFs [40], yet products based off of the patent were not found. Readers are directed towards other articles for additional perspectives on the commercial applications and trends of NFs [41], [42], [43]. Additional sections are included as well, including developments in cellular measurements and others. Each subset of sensors entails their own advantages, such that it is necessary to summarize a range of sensors utilizing NFs for disease detection and monitoring.
There are many fabrication techniques to synthesize NFs. While not the focus of this review, a brief section covering the common methods and usage cases was deemed necessary for clarity. Fabrication methods include drawing [44,45], self-assembly [46,47], and multiple types of spinning methods—including wet spinning [48], [49], [50], dry spinning [51], [52], [53], and electrospinning techniques [54], [55], [56]. There have been some developments towards other, novel methods for the production of fibers, including forcespinning [57,58]. Of the presented methods, electrospinning is the most common method for production on an industrial scale. Electrospinning is a process first described in the 1960s by Taylor [59] and popularized by Reneker [60] in the 1990s, whereby a bias is applied to a polymer solution, which then causes a buildup of charge on the surface of the polymer. When the electrostatic forces overcome the surface tension of the solution, a polymer jet is ejected. This jet is pulled towards a collector—of which there are many configurations—and due to an unstable whipping motion, the fibers are stretched such that micro- or nano- fibers are produced [61], [62], [63]. Controlling this interplay, electrospun polymer fibers span over four orders of magnitude of diameter, with fibers of fewer than 10 polymer molecules up to conventional textile fibers. A diagram of the electrospinning process is shown in Fig. 1. By increasing the number of spinnerets, this process can be easily scaled to meet a variety of production capacities [42,56]. While most work remains at the proof-of-concept stage at this time, an important factor for possible commercial applications is the ease of scalability. While work has been done for the scaling of the electrospinning process, high voltages and many nozzles are often necessary for sufficient yields. Alternative methods of fiber production have been developed with a focus on producing large quantities, including a gas jet process [64] and a shear-driven process [65].
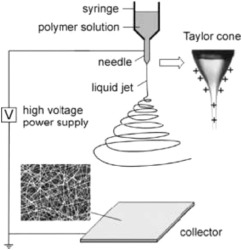
Fig. 1. Schematic of the electrospinning process; the charged polymer solution leaving the needle is shown, the whipping instability process, and the formation of micro- or nano-fibers on the collector. Reproduced with permission [66]. Copyright 2004, John Wiley and Sons Disease and Illness Detection.
Modern healthcare relies on many techniques for the diagnosis of diseases and illnesses, including looking at physical vital signs, symptoms, and also utilizing tests for symptomatic and asymptomatic individuals. These tests, however, may need to be sent to a laboratory, take time to receive results, and are likely expensive to have performed [67]. Functionalized NFs are, therefore an alternative and potential improvement over traditional clinical testing and are capable of selective and sensitive detection due to their high surface area to volume ratio. This allows for enhanced contact between specific biomarkers and the detection receptors. Producing NFs is typically cost-effective, and research is actively being done to detect specific biomarkers at the same levels as traditional testing.
Malaria continues to be a disease that affects millions globally, especially those in tropical and near-tropical regions. In 2015, there were 214 million cases of malaria globally. [68] There are six plasmodial species responsible for the disease, and therapeutic plans differ based on the specific parasite. [69,70] Early and accurate detection of malaria would lead to improved health outcomes for many, and much research has been done applying NFs to the problems of selective and sensitive detection. Before treatment options can be considered, a diagnosis must be performed first. With malaria specifically, many of the regions lack resources to conduct costly testing or have the necessary laboratory equipment—the implementation of NFs may prove an alternative that can be condensed into a portable and inexpensive apparatus capable of being provided to many more people. There has been much work done for the detection of malaria, and an overview of recent advances in the detection of malaria was outlined by Ragavan et al. [5]. The authors also discussed advances in a variety of nanomaterials for malaria detection, focusing on a broader scope than only nanofibers.
In 2014, Gikunoo et al. [69] pioneered a novel method for the early diagnosis of malaria. Here, a secondary antigen, known as PfHRP-2, is the result of the immune response for a specific malaria parasite, Plasmodium falciparum, and was detected in sample solutions. A protein is synthesized by the parasite and released into the bloodstream of the host, and results in the PfHRP-2 antigen in response by the host's immune system. The sensor was capable of both colorimetric sensing and electrical sensing, and a limit of detection (LOD) was 0.01 ng-mL−1 of the antibody through measuring the resistivity. The sensor itself was comprised of carbon nanofibers (CNFs) that were grown on glass micro-balloons, as shown in Fig. 2. The CNFs had an average diameter of 50 nm and several micrometers long. Such a morphology is unique in forming a sphere comprised of nanofibers, condensing the NFs into an overall structure on the microscale. This structure enabled a high probability of antibody binding, which thus allowed for the low detection limit reached by the sensor. This technique is similar to most immunoassay techniques, where an antigen for the antibody is added to the surface, then changes in color or electrical properties are observed when binding occurs. Multiple specific antibodies for different types of malaria pathogens could also be attached—therefore permitting the identification of a specific plasmodial species, which aids in choosing therapeutic options. This research has the potential to be developed as an alternative to traditional malaria screening options. This work, however, was not applied to detection in blood or serum samples, and only buffered solutions were evaluated. Additionally, the synthesis method of growing the CNF forest on the micro-balloons may not scale easily, potentially limiting the commercial development of such a sensor technology.
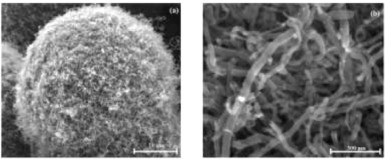
Fig. 2. Scanning electron microscopy (SEM) images of CNF forest on glass micro-balloons. Reproduced with permission [69]. Copyright 2014, CC BY 3.0.
Another sensor for detecting P. falciparum was developed by Brince Paul et al. [71] Here, functionalized zinc oxide NFs were developed through electrospinning and targeted the same analyte as Gikunoo et al. [69], the HRP-2 protein. The LOD was determined to be 6 ag-mL−1 with an impedimetric detection method for the copper doped zinc oxide (ZnO) fibers, which was stated as being the lowest detection limit for malarial parasites reported in the literature. An issue with most ZnO biosensors, however, is a high resistivity from the large band gap present. [72] To address this need, the researchers added copper as a dopant to enhance the conductivity of the NFs alone. After a calcination process to obtain the ZnO fibers, the average diameter was 100–150 nm, while the fibers with copper doping were 150–200 nm, likely due to the addition of copper to the fibers themselves, as confirmed by elemental analysis. SEM images of the NFs are shown in Fig. 3. The sensing mechanism itself is based on an immunoassay technique, whereby a specific antigen is immobilized on the NF surface. Therefore, this work could be expanded to many other immunosensing applications. As the immunoassay is specific to the HRP-2 antigen, good selectivity and sensitivity were observed in complex solutions of other proteins. For scalability, the electrospinning process could easily be modified to address a variety of scales. The researchers developed a new method of functionalizing ZnO NFs and this work could be expanded to the detection of many important biomarkers of interest.
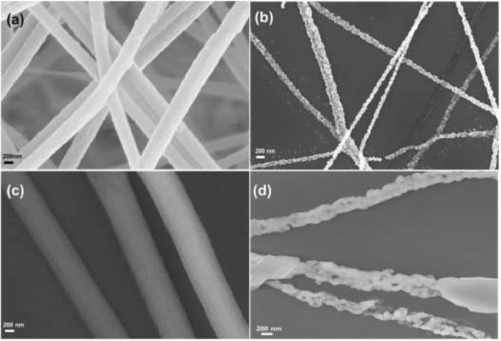
Fig. 3. SEM images of (a) unmodified ZnO NFs before calcination, (b) after the calcination process, (c) ZnO NFs doped with copper before calcination, and (d) ZnO NFs doped with copper after the calcination process. Reproduced with permission [71]. Copyright 2016, Elsevier.
In 2017, Brince Paul et al. [37] continued the work, by making the biosensor for malaria flexible and functionalizing the nanofibers with multi-walled carbon nanotubes (MWCNTs). Similar to the researchers’ previous work, the analyte is the protein HRP-2, which is released into the blood of those infected with the P. falciparum parasite. While the limit of detection is two orders of magnitude higher than the previous work (0.97 fg-mL−1 compared to 6 ag-mL−1 for [71]), this newer sensor is flexible. Instead of doping with copper, MWCNTs are implemented as the conductive material, which allows for the sensor to keep the flexibility of the ZnO nanofibers. Strain measurements could be reported from the sensing apparatus. Under strain, the resistance increased for the sensor, even without the presence of the HRP-2 protein. By measuring relative resistance changes, however, the sensor was still capable of providing readings for the antibody. While this work is similar to the previously reported findings, this sensing apparatus is capable of undergoing strain and is flexible. For wearable platform applications, this flexibility enhances wearability, comfort, and durability of the sensor. Much work has been done into developing sensors with flexible and wearable platforms [73], [74], [75], [76], but the researchers claim that this article presents the first reporting of a flexible, resistive sensor for detection of the HRP-2 protein.
Malaria remains a disease that affects many globally—especially in regions that lack the facilities and infrastructure for quality and effective healthcare. NFs have been implemented to increase the sensitivity of pathogenic detection beyond what is capable with traditional malaria sensing kits. With the ease of scalability of NF processing, commercial applications are likely in development for point of care detection of malaria, which has the potential to improve the quality of life for many. A challenge that remains, however, is that most NF sensing technologies for malaria pathogens remain reliant on immunosensing techniques—and the large-scale production of such antigens will add to the cost of manufacturing for the apparatuses.
Cancer is one of the leading causes of mortality in the modern age, as it is the second most common cause of death in the United States. Generally, cancer is a collection of diseases that are represented by the uncontrollable growth of cells [77]. While the exact causes of cancer are unknown, many contributors throughout a person's lifetime may increase their risk of developing cancer, such as smoking [78,79], excess body fat [80], and exposure to carcinogenic chemical compounds in their surrounding environment [81]. Adequate cancer treatment relies on its identification early, and much work is being done to detect biomarkers for cancerous cells early—which would contribute significantly to improving patient outcomes. Traditional methods for detecting cancer in patients, such as by physical examination, laboratory testing, imaging techniques, or biopsy, are better suited for onset detection of cancer after a tumor is already present [82,83]. Once cancerous cells develop in the body, however, metastasis of the cancerous cells causes further complications and often leads to death; 90% of the deaths from solid tumors are from metastasis to another site [84]. Other perspectives on the topic of cancer detection have been written, including one by Choi et al. [85] that surveys early cancer detection methods through a variety of nanomaterials and another by Chen et al. [86] that surveys applications of electrospinning for all areas of cancer research.
Brince Paul et al. [87] developed a nanowire-based sensor capable of detecting carcinoma antigen-125—a biomarker found on the surface of malignant ovarian cancer cells. Similarly to work done for malaria detection [37], MWCNTs are embedded in ZnO nanowires (similar to NFs). Of note, is the one-pot calcination process that is capable of generating necessary functional groups to allow for the conjugation with the anti-CA-125 antibody. The precursor fibers, before the calcination process, had diameters in the range of 300—400 nm. After the calcination process, thus forming MWCNT-ZnO NFs, the average diameter was reduced to 160—175 nm. The LOD was found to be 0.00113 U-mL−1, as measured in enzyme units of catalytic activity. Acceptable selectivity was observed, even in complex mixtures of other proteins, including immunoglobulin G, bovine serum albumin, histidine-rich protein I, and creatine, as the sensor could still predict the carcinoma-125 antigen concentration. As with similar work, the stability of the proteins functionalized to the NFs is not clearly addressed. Misfolding or denaturing of the antibody in real-world conditions may affect sensor performance. This work, however, could be applied to other biomarkers and demonstrate a cost-effective and sensitive method for the detection of specific target compounds in complex systems. As is, the research presented, if commercialized and expanded, could aid in patient screening and monitoring for ovarian cancer, the leading cause of cancer-related deaths in women.
Wang et al. [39] from Southeast University demonstrated an immunosensor for the detection of tumor suppressor protein p53. The p53 protein is known to be a tumor suppressor protein, and the loss of its activity has been linked to many cell processes in cancerous cells. Of note, the gene that encodes for the suppression of the p53 protein is found in half of all human cancers [88]. Therefore, detection of the p53 protein (AGp53) would enhance early detection efforts for many cancer types. Here, the researchers developed an MWCNT functionalized nylon 6 composite NF (NWCNT-PA6) through an electrospinning process. Then, a sandwich immunoreaction was implemented between the Ab1 capture antibody for AGp53, and horseradish peroxidase (HRP)-labeled detection antibody (HRP@Ab2). Together, the immunocomplex formed generated an amplified signal towards AGp53 by the reduction of polythionine (PTH) with the addition of hydrogen peroxide. A schematic of the immunocomplex formation and sensor function is shown in Fig. 4. The concentration range for AGp53 could be measured from 2 pg-mL−1 to 2 ng-mL−1, and the LOD was found to be 1 pg-mL−1. Similar to other works reported, the MWCNTs were added into the sensor to enhance the conductivity of the sensing apparatus, and the PA6 substrate acted as a semi-conductor, also enhancing the stability and electrochemical response of the sensor. This research could be expanded to additional proteins and biomarkers and demonstrates progress in the amplification of signals for the detection of low analyte concentrations.
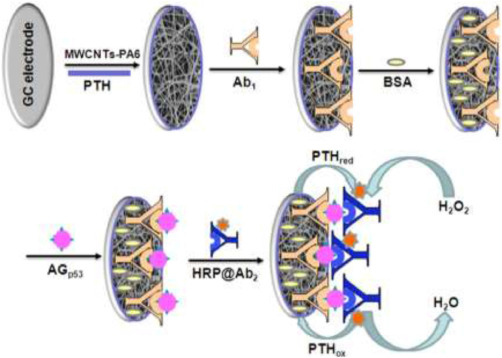
Fig. 4. Schematic of sandwich immunosensor structure with MWCNT and nylon 6 NFs. Reproduced with permission [39]. Copyright 2015, Elsevier.
Contrary to other sensing methods reported, Ding et al. [89] developed a portable sensor for cancerous cells based on pressure detection. First, copper and cobalt -oxide-based NFs were developed to catalyze the reaction from ammonia borane (AB) to hydrogen (H2). This conversion of AB to H2 produces a large increase in gas molecules within the reaction vessel, which therefore increases the pressure within the system. The overall diagram for the sensing apparatus is shown in Fig. 5. The NFs developed through electrospinning had a dual tubular structure, with well-defined inner and outer diameters. The average diameter for the outer tubes was 210 nm, while it was 100 nm for the inner tubes. This structure allowed for the heterojunction catalytic activity of the NFs to facilitate the conversion of AB to H2. The NFs were then modified with PEG-3,4-dihydroxy benzylamine and PEG-3,4-dihydroxy benzyl amine-folic acid to increase dispersion in water and enhance the targeting properties for cancer cells. The presence of folic acid aids in the adhesion of cancer cells to the functionalized NFs in cell lines that overexpress the folate receptors, such as HeLa and KB. The number of cells was correlated to an increase in pressure in the reaction vessel, and the LOD was found to be 50 cells-mL−1. The sensor was also capable of detecting HeLa cells in artificial whole blood samples. The presented approach is a unique one, as the presence of a biomarker, AB, is modified to give a physical parameter that can be measured. Instead of measuring resistance, as most immunosensing work and biomarker detection does, this work builds upon a pre-existing cost-effective pressure sensor. A possible limitation, however, is that the sensor may not function as predicated when the cancer cell lines do not overexpress folate receptors or if healthy cells are producing AB. Other targeting mechanisms may be necessary to facilitate the adhesion of the NFs to specific cell types.
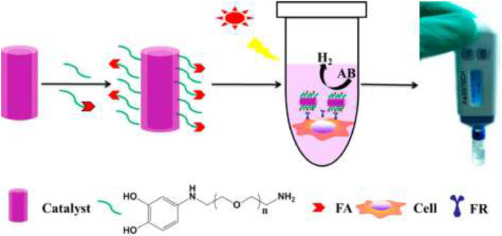
Fig. 5. Schematic detailing functionalization of CuO/Co3O4 NFs to facilitate the conversion of AB to H2. The increase in pressure from the production of H2 can be linked to the number of cells in the culture. Reproduced with permission [89]. Copyright 2017, American Chemical Society.
Another approach taken for the early detection of cancer was performed by Ali et al. [90] through a combination of NFs with a microfluidic chip for breast cancer biomarker detection. Here, a porous graphene foam is modified with titanium dioxide NFs. The human epidermal growth factor receptor (ErbB) family has been previously shown as a biomarker for breast cancer metastasis and diagnosis. Large amounts of the biomarker are often cause for malignant cancers to develop in other parts of the body. A novel structure is presented within the work, as the graphene foam was coated in the NFs through physical adsorption. This modification increased the surface area of the structure, allowing for a greater number of anti-ErbB2 antibodies to be functionalized within the porous structure when compared to the graphene foam material alone. This hierarchical morphology is shown in Fig. 6. The sensor is capable of detection of the ErbB2 antigen over a wide concentration range of 1.0 fM to 0.1 µM. By using the antigen anti-ErbB2, only the specific antibody was recognized, even in the presence of similar biomarkers from the same family of antibodies. Similar to many of the other works, this technique, with the hierarchical structure consisting of pores and NFs, could be expanded for the detection of other biomarkers by changing the antigen that was adhered to the composite material. One challenge with the presented work, however, may be the commercial feasibility of the complex manufacturing to develop the sensor. While the electrospinning process can be performed at many different processing capacities, the development of the graphene foam and integration with a microfluidic device may be difficult to scale to be commercially viable.
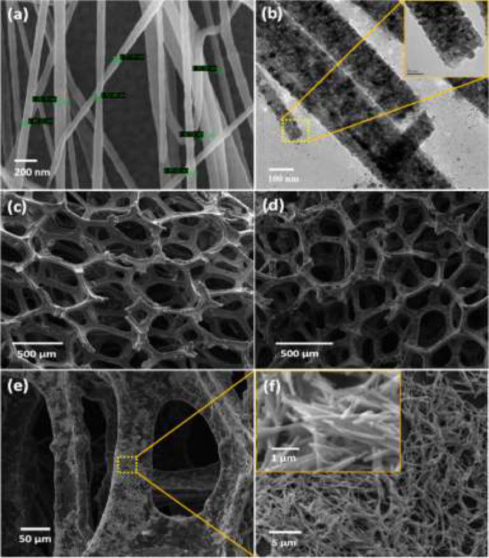
Fig. 6. (a) SEM images of the titanium dioxide NFs, (b) TEM images of NFs, (c) SEM image of graphene foam structure, (d–f) SEM images of the graphene foam with the NFs physically adsorbed. Reproduced with permission [90]. Copyright 2016, American Chemical Society.
Alternatively, to cancer biomarker detection, Shaibani et al. [91] developed a sensor capable of monitoring the metabolism of cancerous cells in real-time. Instead of focusing on cancer cells as the target analytes, metabolites from the cells’ metabolism, specifically the release of lactate, were monitored. Here, pH-sensitive hydrogel NFs composed of poly(vinyl alcohol) and poly(acrylic acid) were used to spatially monitor local changes in pH using a light addressable potentiometric sensor (LAPS). The average diameter of the NFs was 330 nm. The LOD for changes in pH was 0.02 pH using LAPS and the hydrogel NFs. Extracellular acidification can be used to monitor the effects of chemotherapy drugs and to further the understanding of the cancer cells’ metabolism, and this work presents a simple and portable device that can aid researchers in the pursuit of improved anti-cancer drugs.
Another sensor that indirectly improves cancer-related health outcomes was developed by Wang et al. [92], which detects biomarkers related to bacterial infection in cancer patients. Candida albicans often causes high death rates with individuals with cancer, as the fungal infection causes bloodstream and/or organ infection in those with compromised immune systems. Current techniques for the detection of the infection, however, take about five days to report results. Here, a phage was developed to detect the anti-Sap2-igG protein that is produced at low levels during the early onset of the fungal infection. The NF-like phage (approximately 900 nm long and 7 nm wide) is functionalized with magnetic nanoparticles (MNPs), which can then be removed from the solution and biochemically characterized. A diagram of this process is shown in Fig. 7. This work is similar to ELISA methods, but the immunoassay is improved with the rapid replication of the phage and the MNPs on the surface. The LOD of the MNP-assisted phage method is 1.1 pg-mL−1, which is two orders of magnitude below current techniques. The time sampling time was also reduced from about five days to six hours, directly enhancing the time for the treatment of infected immunocompromised patients. Here, a novel approach is implemented, where the resultant NFs are biologically-active phages that demonstrate a morphology similar to NFs.
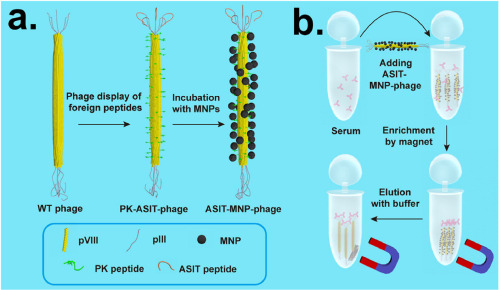
Fig. 7. (a) Showing NF-like phage and functionalization process with MNPs, (b) process for adding modified phages to serum and extraction with a magnet. Reproduced with permission [92]. Copyright 2015, American Chemical Society.
As life expectancies continue to increase, cancer is one of the primary causes of death in the modern age. The plethora of cancer types can make it difficult to detect and accurately diagnose early enough for effective treatment. While physical examinations and tissue cultures will still remain as cornerstones of cancer diagnosis, novel methods that seek to target specific, known biomarkers continue to be active research fields. Recent developments include the addition of MWCNTs and/or NFs to increase the conductivity and surface area of traditional immunosensing assays, therefore improving the sensitivity and detection limits of other methods. Coupled with the selective nature of immunosensing techniques, and the enhanced stability and sensitivity of NFs, these hybrid NF sensors have the potential to improve cancer detection and diagnosis for many.
The advancements of incorporating NFs for detection has benefited other disease diagnoses besides cancer and malaria. While not covered in depth within this review, readers are directed to other sources for a more comprehensive viewpoint. For cardiac biomarker detection, Rezaei et al. highlighted recent advances in the field [93] and a recent work demonstrated detection of a cardiac biomarker with electrospun MWCNTs [38]. Additionally, Eissa et al. produced a NF-based sensor for the detection of Spinal Muscular Atrophy, Cystic Fibrosis and Duchenne Muscular Dystrophy with a disposable electrochemical immunosensor [94]. Other diseases may also be detected through technqiues discussed within this review, but lie outside the main scope of the article and have been sufficiently reviewed in the current literature.
While recent advances directly involving end-users for disease detection has been the primary focus of this review, novel work in vitro is also of importance. Developments for in vitro studies may provide useful information for later in vivo work.
Zhang et al. [95] developed functionalized electrospun NFs for the detection of hydrogen peroxide released from human cancer cells. Hydrogen peroxide was chosen as the analyte of interest because it is a reactive species that causes damage to cells and has been linked to many ailments. Here, an enzyme-free sensor was developed that demonstrated a detection limit of 0.01 µM with a broad linear range of 0.05–5000 µM. The sensitivity was 1241.1 µA-mM−1-cm−2. The authors propose that this is due to the synergistic effects of the composite gold and silver nanofibers, and the high surface-to-volume ratio of the nanofibers. The NFs were produced through an electrospinning process and were bimetallic Au-Ag/Co3O4. This work mitigates many difficulties with hydrogen peroxide sensing by introducing an NF-based sensing method, including short shelf life and high cost of production.
Another area of research for cellular sensing, Kim et al. [96] worked to develop real-time sensing of ascorbic acid in rat liver tissues by using nanowires that were grown on electrospun nanofibers. Ascorbic acid (AA) has been shown to have many health benefits and also has been correlated to the oxidative stress of cells. Here, ruthenium dioxide (RuO2) nanowires were grown on titanium dioxide (TiO2) nanofibers. This was done to balance the expensive ruthenium necessary with an inexpensive to produce backbone structure that would also aid in increasing the surface area of the sensor. The LOD for AA was approximately 1.8 µM and was selective towards AA with differential pulse voltammetry, even in complex biological mixtures. Of note, was the in vitro application of measuring AA in living rat liver tissue. This work could be used to assess oxidative stress on living tissue samples using NFs to increase the sensitivity of the sensor towards AA.
Often, it is desired to measure the amount of oxygen within biological systems directly, but this can prove challenging for in vivo applications. Bhallamudi et al. [97] developed an NF-based paramagnetic probe for real-time detection of oxygen levels in living tissue samples. Here, lithium octa-n-butoxynaphthalocyamine (LiNc- BuO), a well-characterized paramagnetic oxygen sensor, was encapsulated within core-and-sheath nanofibers. The core was composed of polydimethylsiloxane (PDMS) and the sheath was polycaprolactone (PCL), and the NFs were produced through an electrospinning process. The fiber diameter varied between 300 and 500 nm. Electron paramagnetic resonance (EPR) was implemented for the detection of oxygen, and a linear relationship between the EPR linewidth and partial pressure of oxygen was observed. The performance is enhanced over other EPR methods, even with the same LiNc- BuO layer. The authors attribute this improvement in performance to the structure of the NFs—as the high surface area allows for enhanced transport of the oxygen species through the sheath layers to the sensing core. Additionally, this composite NF structure allows for the sensor to be biocompatible and it was shown not to affect cell proliferation nor viability. Here, NFs are implemented to enhance the biocompatibility of the sensor and to improve molecular transport with the associated high surface area to volume ratio.
Cellular measurements are also relevant to disease detection, as much of the drug screening research field characterizes the effects novel compounds have on cells. NFs aid in the generation of accurate and rapid data on cellular chemical species through novel sensors, and therefore improve studies that may result in improved health-related outcomes.
Disease diagnosis and treatment always has and will remain at the forefront of healthcare for all. Earlier detection allows for improved and more effective treatments and significantly improves clinical outcomes. Nanofibers (NFs) are becoming well integrated into a variety of research areas, including applications in immunosensing and disease detection. The large aspect ratios of having nanometer-scale diameters and lengths of several centimeters allow for high surface area to volume ratios. With this, the sensing area of sensors can be improved, often resulting in enhanced selectivity or detection limits of analytes. NFs, coupled with immunosensing techniques, are able to detect biomarkers, including peptides, proteins, and antibodies, at levels lower than other methods, such as ELISA. Therefore, NFs are improving the state of disease monitoring and diagnosis, especially for malaria and cancer, among many others not covered within this review. Looking forward, however, the main challenge facing the novel sensors are the prospects of commercial viability. Little work has been successfully translated from the proof-of-principle scale to industrial production. While NFs can be produced in large scales, the sensors themselves are often complex to manufacture and involve multiple steps. Even then, fiber uniformity and quality control may prove as future concerns for the increased yield of such nanostructures, as there will always remain a balance between manufacturing speed and precision. Additionally, the long-term stability of such systems has generally not been characterized. The end goal for such sensors still has not been met—to produce cost-effective, sensitive, selective, and non-invasive sensors for the accurate detection of diseases. With all of these criteria met, measurably improved health outcomes are likely the result.
Article Source:https://www.sciencedirect.com/science/article/pii/S2666053920300023#sec0007