© Copyright 2020 Foshan Membrane Technology Co., Ltd. All rights reserved. Sitemap
By Bhavna Kaveti Reviewed by Susha Cheriyedath, M.Sc.
Over the years, researchers have tried hard to comprehend topographic signals that promote cell mechanical sensitive responses. The extracellular matrix (ECM) provides a complex cellular microenvironment that controls cellular behavior. Nevertheless, only a few functions of these factors are understood, and most remain obscure.

Study: Curved Nanofiber Network Induces Cellular Bridge Formation to Promote Stem Cell Mechanotransduction. Image Credit: Anusorn Nakdee/Shutterstock.com
An article published in Advanced Sciences presented a convenient method to demonstrate the curved structure of the ECM network that regulates stem cell mechanotransduction. Here, an ECM-mimicking nanofiber network was prepared using electrospinning technology.
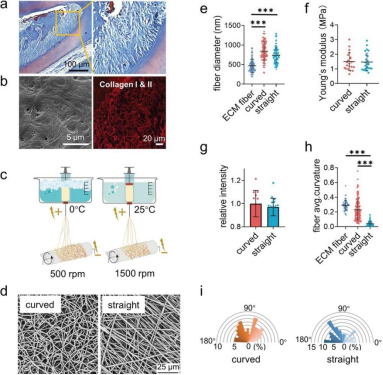
Figure 1. Fabrication and characterization of the curved and straight nanofiber network. a) The Representative images of masson staining of the periodontal tissues. b) The SEM (left) image of the decellularized periodontal ligament tissues and the representative fluorescence image of the collagen I and II (right) in periodontal tissues. c) Scheme of the curved and straight nanofiber network fabrication. The curved and straight fiber network require 0 °C and 25 °C electrospinning temperature, respectively. d) The representative SEM images of the curved and straight fibers (three technical replicates). e) The diameter of the ECM fibers in the periodontal tissues and the artificial fibers (n = 100, two technical replicates). f) Young's modulus of the curved and straight nanofiber network as detected by Nanoindenter (n = 20, two technical replicates). g) Specific surface area of the curved and straight surfaces as detached by the fluorescent intensity of the adsorbed FITC-BSA at 562 nm (n = 12, two technical replicates). h) The average curvature of the ECM fibers in the periodontal tissues and the artificial fibers (n = 160, two technical replicates). i) The orientation angles (n = 100, two technical replicates) of the curved and straight fibers.
The curved nanofiber promoted cell bridge formation due to cytoskeleton tension. Moreover, the myosin-II-based intracellular force generated by the actomyosin filaments inclined the cell lineage towards osteogenic differentiation. Thus, the present study has provided a better understanding of the effects of topographic signals on cell behavior, thereby aiding the development of new biomaterials.
According to recent studies, the physiological and behavioral functions of cells are influenced by biochemical and physical factors. Novel biomaterials that mimic ECM’s stiffness, degradation, ligand diffusion, stress relaxation, and other physical properties, in addition to the usual chemical effects, have been created.
Nanomaterials, such as nanofibers, are mostly fabricated through electrospinning. In this process, a strong electric field is used to transform solution-based polymers into continuous nanometer-sized fibers.
Various nanofibers differ in their properties, including surface-to-volume ratio and morphology. These characteristics can be altered based on the polymer and intended application. The electrospinning parameters, solution parameters, and ambient characteristics affect the properties of the nanofibers.
Stem cells can develop into various cell types and construct any tissue in the body. However, stem cells have low vitality and are challenging to multiply, which limits their application for a wider range of prospective therapeutic benefits.
Stem cells and electrospun nanofibers have two key advantages. First, by changing the chemical characteristics of the nanofibers to enhance their interactions with stem cells, they can operate as advantageous scaffolds for maintaining stem cells. Second, stem cells can be delivered using nanofibers to particular tissues or organs for tissue engineering and wound repair.
Previous reports have suggested that cancer cells unbend the curled collagen fibers in the ECM during tumor growth. Although curved structures in the fibrous connective tissue, known as the periodontal ligament, were previously known, their function at the cellular level remains unclear. Moreover, studies in this area have been restricted by the absence of techniques for creating curved nanofibers.

Figure 2. Cells bridge on the curved fibers. a) The representative displacement fields and b) the displacement quantification of the deformation of the curved and straight fibers under cell traction force as indicated by the embedded fluorescent microbeads (n = 50, two technical replicates). c) The representative images of the F-actin labelled PDLSCs on the curved and straight fibers. d) Analysis of the edge curvature of the representative cells from (c) with MATLAB analysis. e) The plotting of the curvature fluctuation of the complete edge of the representative cells from (c) as analyzed by MATLAB. The curvature greater than 1 was not necessary to be shown. f) The average curvature (n = 50, two technical replicates), g) the vertex number (n = 50, two technical replicates), and h) the percentage of the bridged edge (n = 30, two technical replicates) of the single cells cultured on the curved and straight fibers. The point with curvature greater than 1 is defined as a vertex. i) Schematic of the 2D Laplace's law model adapts to cell non-adhesive bridge. The radius of the curvature of the non-adhesive bridge reflects the balance between the surface tension σ and the linear cell edge tension λ following R = λ/σ. The greater the R, the greater the force at the adhesion point. j) Model predicts the actomyosin traction force as a function of radius (R) and distance (d) as well as k) the effective pressure exerted by contractile bundle as a function of myosin motor density (m(myo)) and angle (φ) at the non-adhesive bridge. Larger R and d lead to the increased traction force, while myosin aggregation contributes to intracellular force. l–n) Quantification of the radius of curvature (R), the distance between the two ends of the arc (d) (n = 20, two technical replicates), and the R/d value of the cells on the curved and straight fibers as well as the cells treated by blebbistatin on the curved fibers. o) Measurements of cellular bridge R and d over a range of linear cell edge tension (λ) consistent with model predictions. Decreased cellular traction force exhibited lower R and d (+blebb represents the cells treated by blebbistatin on the curved fibers).
Despite previous reports on electrospinning technology to fabricate biomaterials that mimic the ECM, only a few reports have described the fabrication of curved nanofibers. On the other hand, other studies that carried out low-temperature electrospinning have focused on the porosity of the matrix rather than the topology of nanofibers.
In this study, cryogenic electrospinning technology was utilized to fabricate ECM-mimicking curved nanofibers as a tool to study cell response when exposed to curved structures. Interestingly, curved nanofibers influenced the behavior of stem cells, altering their adhesive nature compared to straight nanofibers.
While cells adhered along straight nanofibers, they crossed curved nanofibers to form cell bridges, indicating that the cell bodies overhung instead of attaching to the nanofibers.
The formation of cell bridges rearranged the distribution of the actomyosin cytoskeleton and imparted extra intracellular force, enhancing stem cell mechanotransduction and promoting osteogenic differentiation. The new findings of this study helped obtain a better understanding of the crucial role of biomechanical principles in promoting the development of tissue engineering.
Thus, the present investigation of cell mechanosensing revealed that, while the cell boundary was frequently parallel to the surrounding straight nanofibers, it invariably traversed multiple curved nanofibers as bridges. The cells on the curved nanofibers had a significant percentage of unbound borders that formed large radial arcs that bowed inwards.

Figure 3. Immunofluorescence staining displays widely distributed cell bridges in the periodontal ligament. a) The representative fluorescence images of nuclei (blue), F-actin (green), and collagen I (red) staining of the mouse periodontal ligament. b) Canny edge test image of the yellow box area in (a). The magenta and green represent the collagen I and F-actin, respectively. c) The average curvature of the cell edges (n = 50, two technical replicates) of the cells in periodontal ligament and cultured on the artificial fibers.
In summary, a simple electrospinning technology that operates at a low speed and temperature to fabricate ECM-mimicking curved nanofiber structures was developed. While the curved nanofibers promoted discrete adhesion in stem cells, straight networks induced the formation of continuous adhesion by stem cells along with the fiber structure.
The curved nanofibers stimulated stem cell mechanotransduction by forming a cell bridge, thereby promoting osteogenic differentiation and proliferation of stem cells. Inducing mechanotransduction and mechanosensing signaling pathways via the formation of nonadhesive bridges caused actomyosin to aggregate and contract.
Thus, the present study demonstrated that the knowledge of cell mechanosensing and tissue development could be improved by using this curved matrix to enhance the database of biomaterials that mimic the ECM.
Article Source:https://www.azonano.com/news.aspx?newsID=39928